
Luca Hategan, MSc Student, Walters Lab, co-first 1st author
Please join us in congratulating Luca Hategan, MSc student from Walters Lab as he is co-first 1st author in the paper: Role of the histone variant H2A.Z.1 in memory, transcription, and alternative splicing is mediated by lysine modification published in Nature’s Neuropsychopharmacology
https://doi.org/10.1038/s41386-024-01817-2
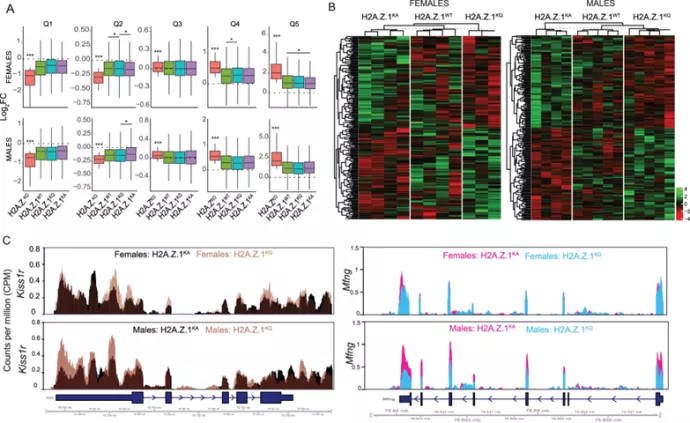
Creating long-lasting memories requires learning-induced changes in gene expression, which are impacted by epigenetic modifications of DNA and associated histone proteins. Post-translational modifications (PTMs) of histones are key regulators of transcription, with different PTMs producing unique effects on gene activity and behavior. Although recent studies implicate histone variants as novel regulators of memory, effects of PTMs on the function of histone variants are rarely considered. We previously showed that the histone variant H2A.Z suppresses memory, but it is unclear if this role is impacted by H2A.Z acetylation, a PTM that is typically associated with positive effects on transcription and memory. To answer this question, we used a mutation approach to manipulate acetylation on H2A.Z without impacting acetylation of other histone types. Specifically, we used adeno-associated virus (AAV) constructs to overexpress mutated H2A.Z.1 isoforms that either mimic acetylation (acetyl-mimic) by replacing lysines 4, 7 and 11 with glutamine (KQ), or H2A.Z.1 with impaired acetylation (acetyl-defective) by replacing the same lysines with alanine (KA). Expressing the H2A.Z.1 acetyl-mimic (H2A.Z.1KQ) improved memory under weak learning conditions, whereas expressing the acetyl-defective H2A.Z.1KA generally impaired memory, indicating that the effect of H2A.Z.1 on memory depends on its acetylation status. RNA sequencing showed that H2A.Z.1KQ and H2A.Z.1KA uniquely impact the expression of different classes of genes in both females and males. Specifically, H2A.Z.1KA preferentially impacts genes involved in synaptic function, suggesting that acetyl-defective H2A.Z.1 impairs memory by altering synaptic regulation. Finally, we describe, for the first time, that H2A.Z is also involved in alternative splicing of neuronal genes, whereby H2A.Z depletion, as well as expression of H2A.Z.1 lysine mutants influence transcription and splicing of different gene targets, suggesting that H2A.Z.1 can impact behavior through effects on both splicing and gene expression. This is the first study to demonstrate that direct manipulation of H2A.Z post-translational modifications regulates memory, whereby acetylation adds another regulatory layer by which histone variants can fine tune higher brain functions through effects on gene expression and splicing.
Luca Hategan is a second year masters student in the Walters lab studying N6-Methyladenosine (m6A) and FTO in the brain, employing a number of molecular and computational techniques, including a novel antibody free m6A detection paradigm. He is the bioinformatician in the Walters/Zovkic labs conducting analysis on RNA-seq, ChIP-seq, ATAC-seq and snRNA-seq datasets.
Anas Reda completed his masters in the Zovkic lab and is currently a PhD/MD candidate at Vanderbilt University.
