
Ian Still
-
E-mail:
-
Mailing Address:
3359 Mississauga Road
Mississauga ON L5L 1C6
Canada
Research
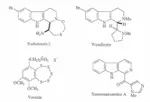
A second major interest is the synthesis of novel types of naturally occurring compounds, particularly those with demonstrated physiological activity. For example, the syntheses of several indole alkaloids from marine organisms, the euchstomins and eudistomidins, have been achieved. These compounds have been demontrated to possess notable antiviral activity as well as in vivo antitumour activity. Eudistomin L, for example, possesses a tetracyclic structure in which ring D is a 1,3,7-oxathiazepine unit: we effected the first synthesis of the unique structural feature by a completely novel approach. We have also developed an efficient route to the closely related alkaloid (-)-woodinine. Our development of new synthetic methods has led us to the synthesis of the unique and potentially important benzopentathiepin, varacin. Very recently we have completed the synthesis of the marine imidazole alkaloid, xestomauzamine A.
Publications
A. Houmam, E.M. Hamed, and I. W. J. Still, A Unique Autocatalytic Process and Evidence for a Concerted-Stepwise Mechanism Transition in the Di iative Electron-transfer Reduction of Aryl Thiocyanates, J. Am. Chem. Soc., 2003, 128,25/8' - 7265.
I.W.J. Still and I.D.G. Watson, An Efficient Synthetic Route to Aryl Thiocyanates from Arenesulfinates, Synth. Commun., 2001, 31(9), 1355-1359.
F.B. Panosyan and I. W. J. Still, An Efficient Route to 5-Iodo-1-methylimidazole: Synthesis of Xestomanzamine A, Can. J. Chem., 2001,79, 11 10-1 114.
I.W.J. Still and J. McNulty, Synthetic Approaches to the Eudistomin Marine Alkaloids, Current Organic Chemistry, 2000, 4(2), pp. 121 -1 38.
I. W. J. Still, R. Natividad-Preyra, and F.D. Toste, A Versatile Synthetic Route to 1,5-Dithiocins from o-Mercapto Aromatic Aldehydes, Can. J. Chem. 1999, 77(1), 113-121.
